Abstract
Introduction
Immune checkpoint inhibitors (ICIs) are approved in multiple indications for cancer care. Most of the clinical trials have not questioned shorter than until disease progression approaches. In this study, we present results from a cohort of multiple advanced cancers treated with restricted anti-PD-(L)1 therapy.
Methods
All patients with advanced cancers treated with anti-PD-(L)1 therapy outside clinical trials at Oulu University Hospital 2014–19 were retrospectively identified from pharmacy records. Clinical variables, treatment history and survival were collected.
Results
106 patients with median age of 66 years with lung cancer (n = 45, 42.5%), melanoma (n = 30, 28.3%), renal and bladder cancers (GU cancers) (n = 26, 24.5%), head and neck (H&N) cancer (n = 4, 3.8%), and colorectal cancer (n = 1, 0.9%) were included in the study. The median (m) OS for the whole population was 14 months (CI 9.7–18.3), 9 months (CI 6.3–11.7) for patients with no IO-free period (n = 64, 62.1%), and 27.0 months (CI 20.6–33.4, p = 0.000001) for patients (n = 39) with IO-free period. The mIO-free survival was 10.0 months (CI 7.1–12.9) for the whole cohort, 8.0 months (CI 1.7–14.3) for lung cancer, 23.0 months (CI 2.6–43.4) for melanoma, and 14.0 months (CI 0.0–20.4) for GU cancer. From the IO-free cohort, 19 patients needed re-treatment during follow-up, of which 8 were re-challenged with anti-PD-(L)1 therapy. The clinical benefit rate of anti-PD-(L)1 re-challenge was 37.5%.
Conclusions
Our study shows that long IO-free periods can be achieved with limited duration of anti-PD-(L)1 therapy with excellent survival outcomes, and that anti-PD-(L)1 re-challenge is feasible in clinical practice.
Similar content being viewed by others
Introduction
Immune checkpoint inhibitors (ICIs) have been approved for treatment of advanced cancers as well as in adjuvant setting for stage III non-small cell lung cancer (NSCLC) after chemoradiation and high-risk resected melanoma (Antonia et al. 2017; Eggermont et al. 2018; Weber et al. 2017). There are currently several anti-PD-(L)1 therapy schemas in various advanced cancers (Balar et al. 2017; Borghaei et al. 2015; Brahmer et al. 2015; Gandhi et al. 2018; Hellmann et al. 2019; Herbst et al. 2016; Horn et al. 2018; Motzer et al. 2018,2015; Reck et al. 2016; Rini et al. 2019; Rittmeyer et al. 2017; Robert et al. 2015a, b) with similar treatment outcomes and toxicity profiles, yet, small subgroups of patients such as advanced NSCLC patients with PD-L1 expression ≥ 50% treated in first line, or tumors with MSI status seem to benefit more from specific therapies.
Due to the unique mechanism of anti-PD-(L)1 therapies, some patients experience long-lasting and durable responses, while a growing data shows that ICI re-challenge can bring meaningful clinical benefit to patients whose anti-PD-(L)1 therapy has been discontinued (Blasig et al. 2017; Fujita et al. 2020; Iivanainen and Koivunen 2019; Niki et al. 2018; Watanabe et al. 2019). Determination of the optimal treatment duration of ICIs has been studied rather minimally. Most of the clinical trials have investigated anti-PD-(L)1 agents until disease progression or severe side effects. One randomized study on advanced NSCLC patients has investigated discontinuing anti-PD-1 therapy at a 1-year fixed time. The results showed that while PFS was inferior for the patients in the discontinued treatment group, the OS was similar (Spigel et al. 2017). Our institutional guideline has restricted the maximal anti-PD-L(1) therapy duration to 6 months in advanced cancers, thus, providing a unique cohort to investigate anti-PD-L(1) therapy with restricted duration. We have previously reported from a small cohort of metastatic cancers that non-inferior outcomes can be achieved with early PD-1 agent discontinuation (Iivanainen and Koivunen 2019).
In this study, we investigate the treatment outcomes of restricted duration of anti-PD-(L)1 therapy with a larger cohort of patients. In addition, we define a novel outcome, immune-oncology (IO)-therapy-free survival (Iivanainen and Koivunen 2019) which captures the unique features of ICI responses, and gives a measure to assess the clinically meaningful benefit of anti-PD-(L)1 treatment.
Materials and methods
Patients
All the cancer patients who had received at least one dose of intravenous anti-PD-(L)1 inhibitor therapy at Oulu University Hospital in 2014–2019 were retrospectively identified from the pharmacy records. Patient’s age, date of diagnosis, date of metastatic disease, TNM staging, histology, molecular status of the tumor, adjuvant/metastatic treatment regimens, treatment-related adverse events, tumor responses, date of progression, and date of death/last follow-up were collected manually from the electronic patient records. Progression-free (PFS) and overall survival (OS) were calculated from the first date of anti-PD-(L)1 treatment to the documented tumor progression, death or end of follow-up (PFS) or to death or end of follow-up (OS). Tumor progression and/or death was counted as an event. Patients whose anti-PD-(L)1 treatment was discontinued in at least stable disease (SD) because of adverse events, complete response (CR), or maximal institutional recommended treatment length (6 months) were subjects to immuno-oncology (IO)-therapy-free survival analysis. IO-therapy-free survival was calculated from the last dose of anti-PD-1 therapy to the next treatment regimen, death or end of follow-up, of which the first two were counted as events. Tumor responses were retrospectively analyzed from electronic health records by two independent investigators (SI and JPK) with 100% concordance.
Data collection was carried out according to national legislation and under a permit from the medical director of Oulu University Hospital (study no. 299/2016). Anonymization was carried out before data analysis. Informed consent was not sought due to the register nature of the study.
Statistical analysis
IBM SPSS Statistics 24.0.0.0 for Windows was applied for statistical analysis. Survival was analyzed by using the Kaplan–Meier method with the log-rank test. Probability values below 0.05 were considered significant.
Results
Demographics
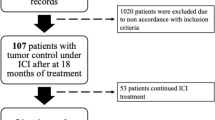
Based on pharmacy records of Oulu University Hospital, a total of 106 patients with advanced stage cancer had received single-anti-PD-(L)1 therapies between August 2014 and August 2019 in outpatient settings and were included in the statistical analysis. The median age of the patients was 66 years and the majority of the patients (65.1%) were male. The cohort included patients with lung cancer (n = 45, 42.5%), melanoma (n = 30, 28.3%), renal and bladder cancers (genitourinary, GU cancer) (n = 26, 24.5%), head and neck (H&N) squamous cell carcinoma (n = 4, 3.8%), and one patient with colorectal cancer (0.9%). Most of the patients (91.5%) had stage IV disease and ECOG 0–1 (98.1%) performance status. Anti-PD-(L)1 therapy was given as a first-line therapy to a majority of the melanoma patients (73.3%), while 31 patients (68.9%) with lung cancer received the therapy in second line. All the other patients received the therapy in second or later line. The detailed demographics are presented in Table 1.
Overall survival of the whole cohort and patients with IO-free period
From the 106 patients identified from the electronic healthcare records, 103 were eligible for survival analysis while three patients had non-assessable treatment response due to recent (< 3 moths) anti-PD-(L)1 therapy initiation. The median follow-up time was 8 months (CI 0–44.0). The overall survival was analyzed for the whole cohort (n = 103) and based on the status of the anti-PD-(L)1 therapy discontinuation. Patients whose anti-PD-(L)1 therapy was discontinued in at least SD response because of adverse events, CR, or maximal institutional recommended treatment length (six months), formed the IO-free cohort (n = 39). The median OS for the whole population was 14 months (CI 9.7–18.3) and for patients with no IO-free period (n = 64, 62.1%), the mOS was 9 months (CI 6.3–11.7), while for patients with IO-free period (37.9%) the median overall survival was 27.0 months (CI 20.6–33.4) (Fig. 1a, p = 0.000001). We also analyzed survival according to disease type based on the IO-therapy-free categorization. Among lung cancer patients, there were 19 patients (48.7%) with IO-free period, whereas 14 (35.9%) melanoma and 6 (15.4%) GU cancer patients had anti-PD-(L)1 therapy discontinued at least in SD at the six months time point. Median OS for the whole lung cancer cohort was 13.0 months and there was a statistical difference (p = 0.0001) between the 19 patients with IO-free period (19.0 months, CI 10.3–15.7) and patients with no IO-free period (8.0 months, CI 2.3–13.7) (Fig. 1b). Median OS for all the melanoma patients was 21.0 months (CI 11.7–30.3). For patients with IO-free period (n = 14), the median OS was 38 months (CI 23.0–53.0), while the overall survival of patients with no IO-free period was much lower, only six months (p = 0.000006) (Fig. 1c). Probably due to the rather low number of GU cancer patients with IO-free period (n = 6), the subgroup analysis was statistically non-significant (Fig. 1d). Patient demographics of the IO-free survival cohort are presented in Table 2.
IO-therapy-free survival
Patients who had at least SD response after six months of anti-PD-(L)1 therapy initiation were included in the IO-therapy-free survival analysis. The IO-free survival was defined as the length of the time from the last infusion of anti-PD-(L)1 therapy to the initiation of next treatment regimen, death or end of follow-up, the first two counted as events. The characteristics of the patients whose anti-PD-(L)1 therapy was discontinued in clinical response are presented in Table 3. Anti-PD-(L)1 therapy was discontinued in majority of the patients (71.8%) because of the maximal institutional-recommended treatment duration, whereas adverse events were counted for ~ 25% of the therapy discontinuations. Median duration of ICI therapy was 3.0 months and at the time of therapy discontinuation, five patients had CR (12.8%), 10 PR (25.6%), and 24 SD (61.6%) as disease status. With median follow-up time of 5 months (CI 0–34.0), the median IO-free survival was 10.0 months (CI 7.1–12.9) for the whole cohort, 8.0 months (CI 1.7–14.3) for lung cancer, 23.0 months (CI 2.6–43.4) for melanoma patients, and 14.0 months (CI 0.0–20.4) for GU cancer (Fig. 2a–d).
Re-treatment of the IO-free cohort
During the follow-up period, 16 patients (41.0%) from the IO-free cohort had no need for further therapy initiation. Re-treatment modalities for patients (n = 19, 48.7%) whose disease required re-treatment included anti-PD-(L)1 therapy re-challenge (n = 8, 42.1%), palliative radiotherapy (n = 7, 36.8%), chemotherapy (n = 3, 15.8%), and tyrosine kinase inhibitor therapy (n = 1, 5.3%). Four patients died without any further therapy. After the anti-PD-(L)1 re-challenge, the response rates included one PR (lung cancer) (12.5%), two SD (25.0%) (GU cancer, melanoma), and five PD (62.5%) (three melanoma patients and two lung cancer patients). There was no correlation between the initial response to anti-PD-(L)1 therapy and re-challenge response. The patients with clinical benefit on the re-challenge had PR (n = 2) or CR (n = 1) as initial response.
Discussion
By far, ICI monotherapies have changed the treatment landscape of many advanced cancers with durable and even complete responses with acceptable toxicity profile. However, ICIs create a substantial economic challenge due to the undefined benefitting patient pool and treatment duration. The response rates to ICI monotherapies are generally low ~ 10–30% in undefined populations and there is a lack of clinically relevant predictive biomarkers to enrich the benefitting population. Furthermore, the optimal treatment duration in responding patients remains to be studied, since the registration trials have investigated the use of these agents until PD or up to 2 years.
In the current study, we present real-world treatment outcomes from a cohort of over 100 advanced cancer patients treated with restricted duration of anti-PD-(L)1 therapy. We have previously reported outcome results in the same setting with limited number of cases and a short follow-up time generating uncertainties in the data. Our previous results suggested that the limited treatment length of anti-PD-(L)1’s is associated with a low risk of rapid disease progression after therapy discontinuation, and with excellent survival outcomes of the approach. Currently, there is only a single study that has investigated anti-PD-1 therapy discontinuation in response in randomized fashion. The results of this study suggested that therapy discontinuation increases the risk for disease progression, but does not worsen the survival. Even though this study is generally thought to be negative for anti-PD-1 therapy discontinuation in response due to PFS difference, we feel that overall survival should be the primary end point of a discontinuation study in the context of metastatic cancer. There is a very limited number of retrospective studies on restricted anti-PD-(L)1 therapy, some of these, however, suggesting the feasibility of the approach (Jansen et al. 2019). Nevertheless, there are data from prospective trials indicating that patients can experience ongoing benefit after treatment discontinuation also in the absence of PD or treatment-related toxicities (Long et al. 2018; Robert et al. 2018; Topalian et al. 2014). There is a strong need for high-quality evidence to define early stopping rules; hence, two prospective trials, STOP-GAP (NCT02821013) and DANTE (ISRCTN15837212), are recruiting metastatic melanoma patients to evaluate the optimal treatment duration and the role of re-challenge of anti-PD-1 therapy.
The major finding of our study is that median IO-free survival of patients is 10.0 months (CI 7.1–12.9), which is very close to our previous report of 12.0 months (CI 3.5–20.5). This generates further cumulating evidence that restricted anti-PD-(L)1 duration does not lead to a rapid disease progression after therapy discontinuation. Since we had a larger cohort of patients with therapy discontinuation, we were able to study IO-free survival also in various cancers. The median IO-free survival ranged from 23.0 with melanoma patients to eight months in the lung cancer cohort, reflecting disease-specific nature to anti-PD-(L)1 response. This is in line with previous works showing that progression-free survival after IO therapy discontinuation is inferior in lung cancer compared to melanoma (Jansen et al. 2019; Spigel et al. 2017).
In our cohort, over 60% of patients had SD as the best response before treatment discontinuation. Many have suggested that treatment discontinuation is feasible in CR, but whether this can be generalized into patients with other types of responses, is unknown. Our study suggests that treatment discontinuation is a viable option also in PR and SD responses. The median duration of anti-PD-(L)1 therapy in our cohort was three months, which might reflect the rather high incidence of irAEs leading to preliminary treatment discontinuation. Yet, in a registration trial of pembrolizumab in advanced melanoma, the median duration of therapy was six months, while the median time to objective response was under three months (Robert et al. 2018). In this study, the majority of the patients with CR as their best confirmed overall response had PR or CR already at their first radiologic response assessment, implying that treatment benefit could be evaluated already in the early course of therapy.
Compared to our previous report, we now had more follow-up time and data available on the patients who had progressed within the IO-free period. 49% of the patients required re-treatment after IO-free period, and 42% of these were re-challenged with anti-PD-(L)1 therapy. There was a clinical benefit rate of 38% in patients with anti-PD-(L)1 re-challenge, suggesting that patients can respond to anti-PD-(L)1 re-treatment. Our results mirror other RWD results of anti-PD-(L)1 re-challenge where disease control rate has varied from ~ 20% to a bit over 40% with NSCLC patients (Fujita et al. 2020; Niki et al. 2018; Watanabe et al. 2019). A recent retrospective study on melanoma patients treated mainly outside clinical trials, whose anti-PD-1 treatment had been discontinued for any reason, reported a response rate of only 14.7% of single-anti-PD-1, and ~ 25% to combination-ICI therapy re-challenge, while there was no causality between the initial best overall response to re-treatment response (Betof Warner et al. 2020). Since improved survival is the primary end point of oncological care, strong conclusions cannot be made on the low response rates of therapy re-challenge, especially considering the unique nature of immunotherapy responses which generally do not follow traditional oncological response measures.
Analysis for the overall survival suggested that even though anti-PD-(L)1 therapy length was restricted, the overall survival was not sacrificed. In our study, the median OS for the melanoma cohort was 21.0 months, which is in line with other published RWD studies of advanced melanoma patients treated in first line (Grude et al. 2019). Furthermore, our NSCLC cohort consisted mostly of patients treated with anti-PD-1 therapy in the second line with the median OS of 13.0 months. This follows closely the survival results of nivolumab arm (median OS of 12.2) in the registration trial Checkmate-057 (Borghaei et al. 2015). Expectedly, the survival for the patients with IO-free period was significantly better than for those without it. However, it would demand a randomized prospective trial setting to be able to make strong conclusions on the restricted therapy length to survival.
Financial toxicity is a growing problem in the field of oncology, especially through the wide-scale use of anti-PD-(L)1 therapies. Our study shows that long IO-free periods can be achieved with limited duration of anti-PD-(L)1 therapy with excellent survival outcomes. This makes a strong statement that the anti-PD-(L)1 therapy length of registration trials should be questioned and investigated.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on request.
References
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, PACIFIC Investigators et al (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377(20):1919–1929. https://doi.org/10.1056/NEJMoa1709937
Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T et al (2017) First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 18(11):1483–1492. https://doi.org/10.1016/S1470-2045(17)30616-2
Betof Warner A, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA et al (2020) Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. JCO. https://doi.org/10.1200/JCO.19.01464
Blasig H, Bender C, Hassel JC, Eigentler TK, Sachse MM, Hiernickel J et al (2017) Reinduction of PD1-inhibitor therapy: first experience in eight patients with metastatic melanoma. Melanoma Res 27(4):321–325. https://doi.org/10.1097/CMR.0000000000000341
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S et al (2018) Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378(19):1789–1801. https://doi.org/10.1056/NEJMoa1802357
Fujita K, Yamamoto Y, Kanai O, Okamura M, Hashimoto M, Nakatani K et al (2020) Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer 11(1):15–18. https://doi.org/10.1111/1759-7714.13241
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, KEYNOTE-189 Investigators et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005
Grude F, Travers M, Moiteaux B, Beneton N, Jacobzone C, Corre Y et al (2019) Nivolumab and Pembrolizumab in advanced cutaneous melanoma: Results from real-life data in patients from France—evaluation of use, current practices and medico economic approach. J Clin Oncol 37:e21009. https://doi.org/10.1200/JCO.2019.37.15
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E et al (2019) Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381(21):2020–2031. https://doi.org/10.1056/NEJMoa1910231
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, IMpower133 Study Group et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229. https://doi.org/10.1056/NEJMoa1809064
Iivanainen S, Koivunen JP (2019) Early PD-1 therapy discontinuation in responding metastatic cancer patients. Oncology 96(3):125–131. https://doi.org/10.1159/000493193
Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L et al (2019) Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol 30(7):1154–1161. https://doi.org/10.1093/annonc/mdz110
Long GV, Schachter J, Ribas A, Arance AM, Grob J, Mortier L et al (2018) 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. JCO 36(15):9503. https://doi.org/10.1200/JCO.2018.36.15suppl.9503
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, CheckMate 025 Investigators et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813. https://doi.org/10.1056/NEJMoa1510665
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, CheckMate 214 Investigators et al (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378(14):1277–1290. https://doi.org/10.1056/NEJMoa1712126
Niki M, Nakaya A, Kurata T, Yoshioka H, Kaneda T, Kibata K et al (2018) Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget 9(64):32298–32304. https://doi.org/10.18632/oncotarget.25949
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, KEYNOTE-024 Investigators et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D et al, KEYNOTE-426 Investigators (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127. https://doi.org/10.1056/NEJMoa1816714
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J et al, OAK Study Group (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L et al (2015a) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. https://doi.org/10.1056/NEJMoa1412082
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, KEYNOTE-006 investigators et al (2015b) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372(26):2521–2532. https://doi.org/10.1056/NEJMoa1503093
Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM et al (2018) Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol 36(17):1668–1674. https://doi.org/10.1200/JCO.2017.75.6270
Spigel DR, McLeod M, Hussein MA, Waterhouse DM, Einhorn L, Horn L et al (1297ORandomized) 1297ORandomized results of fixed-duration (1-yr) vs continuous nivolumab in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol. https://doi.org/10.1093/annonc/mdx380.002
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32(10):1020–1030. https://doi.org/10.1200/JCO.2013.53.0105
Watanabe H, Kubo T, Ninomiya K, Kudo K, Minami D, Murakami E et al (2019) The effect and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer. Jpn J Clin Oncol 49(8):762–765. https://doi.org/10.1093/jjco/hyz066
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, CheckMate 238 Collaborators et al (2017) Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377(19):1824–1835. https://doi.org/10.1056/NEJMoa1709030
Acknowledgements
Open access funding provided by University of Oulu including Oulu University Hospital.
Funding
This work was supported by Oulu University and Finnish Cancer Institute.
Author information
Authors and Affiliations
Contributions
AT, SI, and JPK contributed to the conception and design of the study, acquired the data, analyzed the data and interpreted the data. AT, SI, and JPK contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval and Informed consent
According to national legislation, informed consent is not needed due to the retrospective and non-interventional nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tikkanen, A., Iivanainen, S. & Koivunen, J.P. Treatment discontinuation and re-initiation of anti-PD-(L)1 agents in metastatic cancers. J Cancer Res Clin Oncol 146, 2153–2160 (2020). https://doi.org/10.1007/s00432-020-03217-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03217-7